By L. Alex Ambrose, DO and Katie Smithwick, PharmD
VCU Health Psychiatry
Richmond, VA
Background
In the late 1950s, clozapine was developed and considered a breakthrough, given its antipsychotic properties without significant extrapyramidal side effects. Unfortunately, in the late 1970s in Europe, a small number of patient deaths occurred due to severe blood dyscrasias, which led to clozapine being pulled from the market. After demonstrating superior response rates in clinical trials with the standard of care at that time, clozapine was approved for treatment resistant psychotic disorders. Later, approval was added for use of clozapine for reduction in risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder. The approval required blood monitoring parameters.
Initially, there were independent registries that were maintained by clozapine manufacturers, but ultimately the FDA launched a shared Clozapine REMS Program in 2015. Multiple iterations of the REMS program followed along with issues with implementation. The REMS program, while well intentioned, created barriers to accessing clozapine that were eventually acknowledged by the FDA.
We know that clozapine is underutilized, likely due to the burden of monitoring and lack of clinical support to ensure monitoring is occurring. This burden was compounded in the US by REMS, due to the reporting requirements and elements preventing dispensing of clozapine by pharmacies. Thankfully, advocacy efforts are tackling this epidemic of underutilization. Advocacy by psychiatrist- and pharmacist- led organizations eventually resulted in the FDA re-evaluating and eliminating REMS on February 24, 2025.
REMS Changes
In its review, the FDA was unable to determine if the REMS program had a significant impact on mitigation of severe neutropenia. They recognized that the REMS program did place a large burden on patients, their families, and clinicians. The elimination of the REMS signals the FDA’s acknowledgement that clinician monitoring for severe neutropenia is sufficient without additional administrative barriers to ensure safe use of clozapine.
What does this mean?
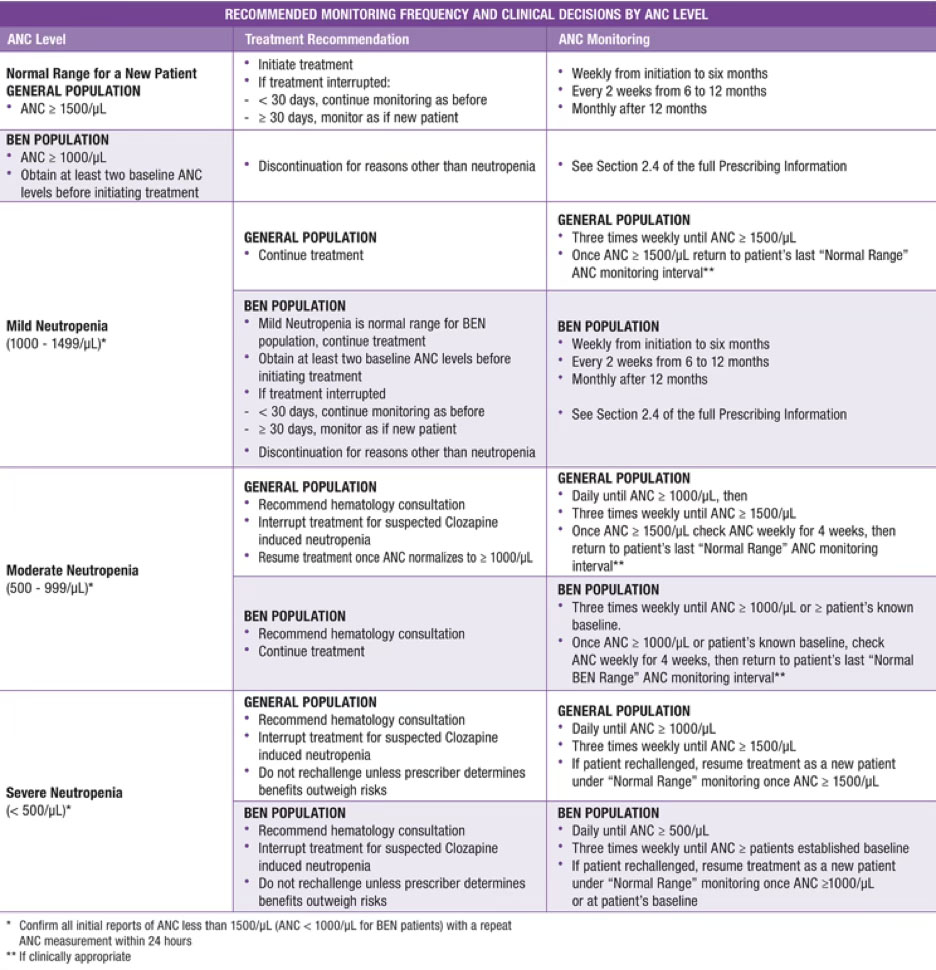
The risk of severe neutropenia is thought to be the highest in the first 18 weeks of treatment, and after years of treatment may approximate the risk with other antipsychotics. Despite changes to the clozapine REMS program, the FDA has not currently expressed any intention to change product labeling regarding monitoring for neutropenia. With the ending of clozapine REMS, the monitoring responsibility falls on prescribers, and best practices are to continue to monitor patients’ ANCs per prescribing guidelines (pictured below taken from manufacturer). Additional research will be helpful to determine the optimal long term monitoring schedule for patients who have been stable without neutropenia on clozapine for more than 2 years.
An added hope of eliminating REMS is that it will help to shift the focus on adverse effect monitoring to include other risks of clozapine that warrant attention, such as myocarditis/cardiomyopathy, gastrointestinal hypomotility, and metabolic effects. This along with the lowering barriers to prescribing will hopefully improve safe use and allow for increased utilization among patients with treatment resistant schizophrenia who were not previously offered a trial of clozapine.
- Leung JG, de Leon J, Frye MA, Singh B, Cotes RO, McElroy SL. The Modernization of Clozapine: A Recapitulation of the Past in the United States and the View Forward. J Clin Psychopharmacol. 2022 Nov-Dec 01;42(6):565-580.
- Northwood K, Myles N, Clark SR, Every-Palmer S, Myles H, Kisely S, Warren N, Siskind D. Evaluating the epidemiology of clozapine-associated neutropenia among people on clozapine across Australia and Aotearoa New Zealand: a retrospective cohort study. Lancet Psychiatry. 2024 Jan;11(1):27-35. doi: 10.1016/S2215-0366(23)00343-7. Epub 2023 Nov 28. PMID: 38040009.
- Richmond, L., & Moran , M. (2025, April 1). FDA Has Ended the Clozapine REMS. What Happens Now?. Psychiatric News. https://psychiatryonline.org/doi/10.1176/appi.pn.2025.04.4.22
- Verdoux H, Bittner RA, Hasan A, et al. The time has come for revising the rules of clozapine blood monitoring in Europe. A joint expert statement from the European Clozapine Task Force. Eur Psychiatry. 2025 Jan 10;68(1):e17.